Trikafta* (elexacaftor/tezacaftor/ivacaftor; ivacaftor) for sale – Buy Trikafta* (elexacaftor/tezacaftor/ivacaftor; ivacaftor) Online
What is Trikafta (elexacaftor/tezacaftor/ivacaftor)* for?
Trikafta (elexacaftor/tezacaftor/ivacaftor)* is a medication used in combination with ivacaftor 150 mg tablets for people with cystic fibrosis (CF) aged 12 years and older with at least one copy of the F508del mutation.
 How effective is Trikafta (elexacaftor/tezacaftor/ivacaftor)* for cystic fibrosis?
How effective is Trikafta (elexacaftor/tezacaftor/ivacaftor)* for cystic fibrosis?
The approval of Trikafta (elexacaftor/tezacaftor/ivacaftor)* for CF was based on two clinical studies.
Study 1
Included 403 people with CF, 12 years and older, with one copy of the F508del mutation and a mutation defined in the study. Trikafta was compared with placebo (a dummy treatment).
According to the study results published:
After 24 weeks, lung function (FEV1) improved significantly.
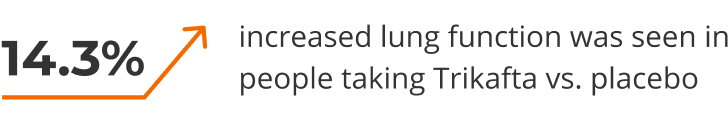
Study 2
Included 107 people with CF, 12 years and older, with two copies of the F508del mutation. Trikafta was compared with ivacaftor and tezacaftor (Symdeko).
According to the study results published:
There was significant improvement in lung function (FEV1).

For more details refer to the prescribing information at the bottom of the page.
 Which side effects can I expect with Trikafta (elexacaftor/tezacaftor/ivacaftor)*?
Which side effects can I expect with Trikafta (elexacaftor/tezacaftor/ivacaftor)*?
According to the studies published ,the main common side effects of Trikafta (which may affect more than 1 in 10 people) in people with CF aged 12 years and older include headaches, upper respiratory tract infections, abdominal pains, diarrhea and rashes.
Please note this is not intended to be a comprehensive guide. Consult your treating doctor and the prescribing information at the bottom of the page for full details of side effects.
How is Trikafta (elexacaftor/tezacaftor/ivacaftor)* taken?

Administration: in the morning take two Trikafta tablets. In the evening, take one ivacaftor tablet. Take these tablets approximately 12 hours apart.
Dosage: 150 mg ivacaftor, 100 mg tezacaftor and 200 mg elexacaftor in the morning and 150 mg ivacaftor in the evening.
Each dose must be taken with fat-containing food.
Please note this is not intended to be a treatment plan. For a personalised treatment plan, consult your doctor. For more details, you can also reference the full prescribing information at the bottom of the page.
Frequently Asked Questions about Trikafta (elexacaftor/tezacaftor/ivacaftor)*
How does Trikafta (elexacaftor/tezacaftor/ivacaftor)* work?
What are some contraindications to taking Trikafta (elexacaftor/tezacaftor/ivacaftor)*?
What is the most important information you should know about Trikafta (elexacaftor/tezacaftor/ivacaftor)*?
Where has Trikafta (elexacaftor/tezacaftor/ivacaftor)* been approved?
Downloads
References
1. Full prescribing information [FDA]: Trikafta (elexacaftor/ivacaftor/tezacaftor) [PDF].
Vertex Pharmaceuticals Incorporated, Oct 2019.
2. FDA approves new breakthrough therapy for cystic fibrosis.
U.S. Food and Drug Administration. 21/10/2019 (last update: 21/10/2019), cited on 10/01/2020
3. Kaftrio product page.
European Medicines Agency, last checked on Sept 2, 2020.
4. Vertex Announces FDA Approvals of Trikafta (elexacaftor/tezacaftor/ivacaftor and ivacaftor), Symdeko (tezacaftor/ivacaftor and ivacaftor) and Kalydeco (ivacaftor) for Use in People With CF With Certain Rare Mutations.
Press release, Dec 21, 2020.
*Trikafta is a registered trademark by Vertex Pharmaceuticals Incorporated.






Reviews
There are no reviews yet.