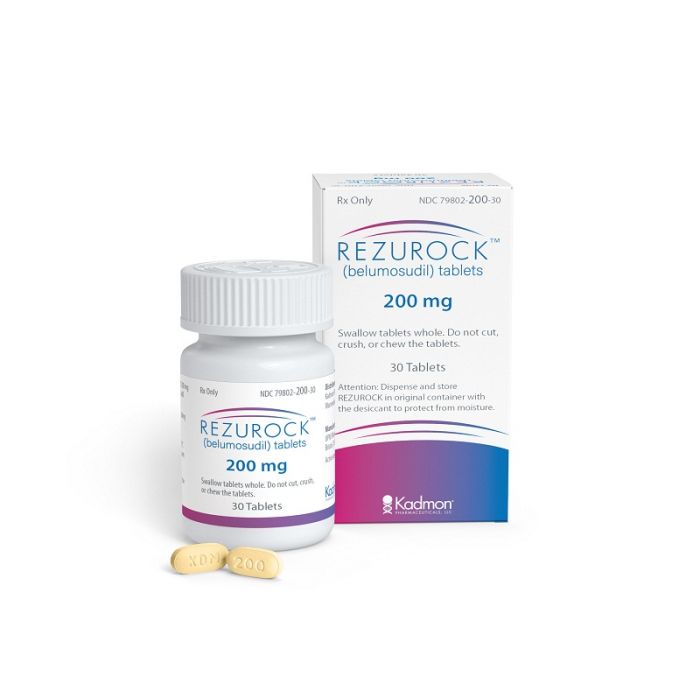
Rezurock (belumosudil) for sale – Buy Rezurock (belumosudil) for sale Online
What is Rezurock (belumosudil) for?
Rezurock (belumosudil) is a kinase inhibitor indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (cGVHD) after at least two prior lines of systemic therapy have failed.[1]
It is available in tablet form, each containing 200 mg belumosudil.[1]
How does Rezurock (belumosudil) work?
In cGVHD, immune cells from the transplanted tissue (graft) attack the patient’s cells (host), which leads to inflammation and fibrosis. Fibrosis may function as a wound-healing process, but in cGVHD, it is the end stage of the inflammatory process. In cGHVD, fibrosis can affect joints or extensive skin areas, which causes the disorder to worsen. Fibrosis may lead to severe life-threatening chronic breathing problems if it affects the lungs.2,3
The active ingredient in Rezurock, belumosudil, is a selective inhibitor of the protein Rho-associated coiled-coil kinase 2 (ROCK2). ROCK is an enzyme that modulates inflammatory response and fibrotic processes.[2,3]
By inhibiting ROCK2, Rezurock may prevent and reduce cGVHD-induced fibrosis.[2,3]
Where has Rezurock (belumosudil) been approved?
Rezurock (belumosudil) was approved for the treatment of people with cGVHD by:
- The Food and Drug Administration (FDA), USA on July 16, 2021.[4]
Rezurock (belumosudil) was approved under Priority Review and was previously granted Breakthrough Therapy designation by the FDA. The FDA reviewed the New Drug Application (NDA) under the Real-Time Oncology Review (RTOR) pilot program.[4]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Rezurock (belumosudil) taken?
The standard dosage is:[1]
- 200 mg taken orally once a day with food.
During the course of treatment, total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) should be measured at least monthly.[1]
In case of adverse reactions, the doctor may decide to reduce the dose of Rezurock (belumosudil).[1]
When this medicine is taken together with strong CYP3A inducers or proton pump inhibitors, the dosage should be increased to 200 mg twice daily.[1]
Complete information about Rezurock (belumosudil) dosage (modifications) and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Rezurock (belumosudil)?
Common adverse reactions
The most common side effects ( ≥20% of patients) listed in the prescribing information include:[1]
- infections
- tiredness or weakness
- nausea
- diarrhea
- shortness of breath
- cough
- swelling
- bleeding
- stomach (abdominal) pain
- muscle or bone pain
- headache
- high blood pressure
Use in a specific population
Rezurock (belumosudil) can be fatal for a fetus, it is advised to avoid pregnancies and breastfeeding. Females who can become pregnant and males with female partners who can become pregnant should use effective birth control during treatment, and for at least 1 week after the last dose.[1]
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.[1]
References
1. Full prescribing information [FDA]: Rezurock (belumosudil) [PDF]
Kadmon Holdings, Jul 2021
2. About belumosudil
Kadmon.com, cited Aug 2021
3. Treating chronic GVHD-induced fibrosis?
Socie, G. et al., Blood, 2018
4. U.S. FDA Grants Full Approval of REZUROCK™ (belumosudil) for the Treatment of Patients with Chronic Graft-Versus-Host Disease (cGVHD)
Kadmon press release, Jul 2021



Reviews
There are no reviews yet.