Crysvita for sale – Buy Crysvita online
What is Crysvita (burosumab) for?
Crysvita (burosumab) is indicated for the treatment of:
- X-linked hypophosphatemia (XLH).
- Tumor-induced osteomalacia (TIO)
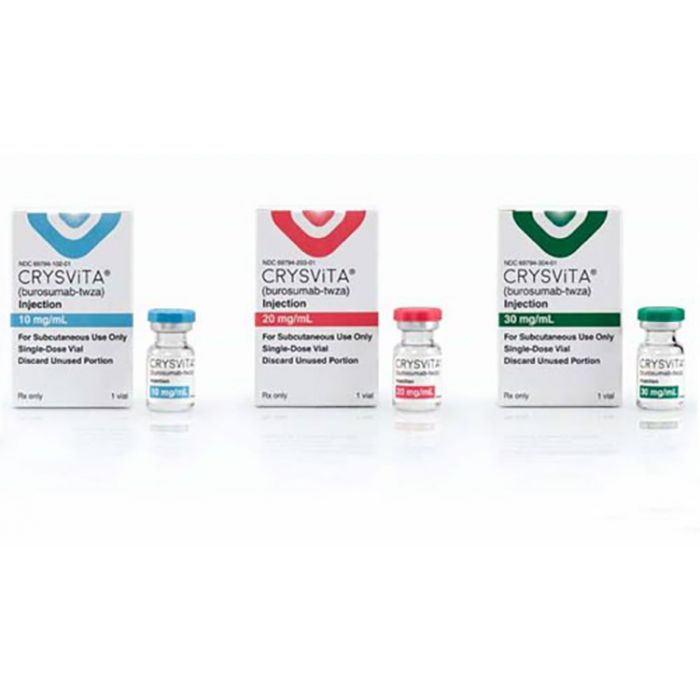
Approved presentations:
- 10 mg/mL in single dose vial
- 20 mg/mL in single dose vial
- 30 mg/mL in single dose vial
Where has Crysvita (burosumab) been approved?
Crysvita (burosumab) has been approved for the treatment of:
- X-linked hypophosphataemia (XLH) in adult and pediatric patients 6 months of age and older, by
- The Food and Drug Administration (FDA) on April 17, 2018.
- The European Medicines Agency (EMA) on February 19, 2018.
- Health Canada on December 06, 2018.
- Tumor-induced osteomalacia (TIO), by
- The FDA on June 18, 2020.
- Health Canada on September 07, 2021.
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about it’s approval in a specific country feel free to contact our support team.
References
Please see full prescribing information and available presentations at:
1. Prescribing Information (FDA): Crysvita (burosumab) [PDF], Kyowa Kirin, 2018.
2. Summary of Product Characteristics (EMA): Crysvita (burosumab) [PDF], Kyowa Kirin, 2018
This content has been reviewed by a Medical Doctor.


Reviews
There are no reviews yet.