Cabenuva (cabotegravir + rilpivirine) for sale – Buy Cabenuva (cabotegravir + rilpivirine) Online
What is Cabenuva (cabotegravir + rilpivirine) for?
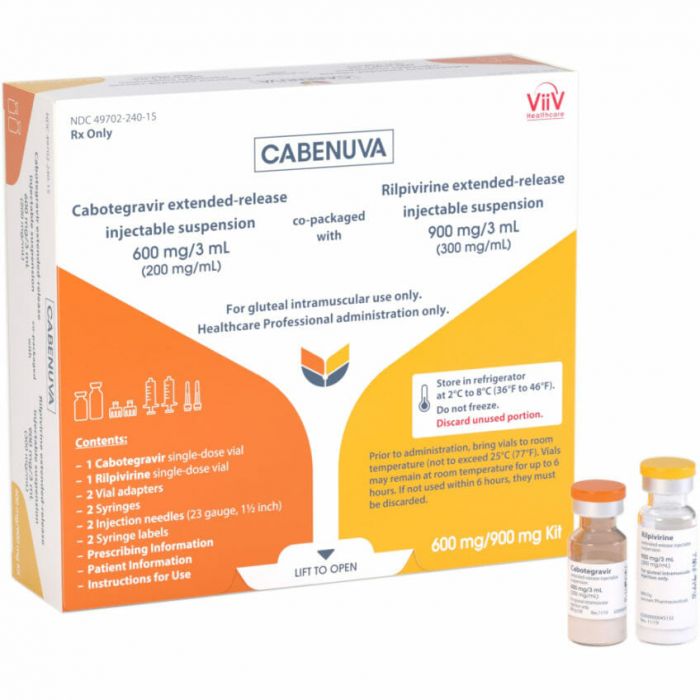
Cabenuva (cabotegravir + rilpivirine) is a prescription medicine used to treat people with human immunodeficiency virus type 1 (HIV-1) infection as a replacement for their current HIV-1 treatment when their healthcare provider determines that they meet certain requirements.[1]
It is available as a vial of cabotegravir suspension and a vial of rilpivirine suspension (400/600mg or 600/900mg).[1]
How does Cabenuva (cabotegravir + rilpivirine) work?
The active ingredient in Cabenuva, cabotegravir, is an integrase inhibitor. It can block an enzyme called integrase, which the virus uses to replicate in the body. By blocking integrase, cabotegravir lowers the number of HIV particles in the blood and keeps the levels low.[2]
The other active ingredient in Cabenuva, rilpivirine, is a so-called non-nucleoside reverse-transcriptase inhibitor (NNRTI). It is a type of molecule that can block the enzyme reverse transcriptase (RT), which is produced by the virus to make new copies of itself. By blocking RT), rilpivirine can lower the number of HIV particles.[2]
The medicine cannot cure HIV infection or AIDS, but it can allow the immune system to repair itself and prevent further damage.[2]
Where has Cabenuva (cabotegravir + rilpivirine) been approved?
Cabenuva (cabotegravir + rilpivirine) was approved for the treatment of people with HIV-1 infection by:
- Health Canada on March 18, 2020.[3]
- The Food and Drug Administration (FDA), USA on January 21, 2021.[2]
- Therapeutic Goods Administration (TGA), Australia on February 23, 2021.[4]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Cabenuva (cabotegravir + rilpivirine) taken?
The standard dosage is:[1]
- The first one month of once-daily starter pills before beginning treatment to assess tolerability to Cabenuva: one 30-mg tablet of Vocabria (cabotegravir) and one 25-mg tablet of Edurant (rilpivirine)
- On the last day of Month 1, patients will be given their first injections: 600 mg (3 mL) of cabotegravir and 900 mg (3 mL) of rilpivirine
- Starting in Month 3, continuation injections will start: 400 mg (2 mL) of cabotegravir and 600 mg (2 mL) or rilpivirine will be given once monthly.
The 2 injections are given one into each side of your buttocks, and must be given by a healthcare professional.[1]
Cabenuva (cabotegravir + rilpivirine) may affect the way other medicines work, and other medicines may affect how this medicine works, so it is important that you inform your doctor about all medicines you take. The medicine may remain in your blood for prolonged periods (up to 12 months or longer).[1]
Complete information about Cabenuva (cabotegravir + rilpivirine) dosage (modifications) and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Cabenuva (cabotegravir + rilpivirine)?
Common adverse reactions
The most common side effects ( ≥2% of patients) listed in the prescribing information include:[1]
- Injection site reactions
- Fever (pyrexia)
- Tiredness (fatigue)
- Headache
- Muscle pain
- Nausea
- Sleep disorders
- Dizziness
- Rash
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Serious injection site reactions
- Liver problems
- Depression or mood changes
Use in a specific population
It is not known if Cabenuva (cabotegravir + rilpivirine) can harm a fetus. The medicine may remain in your blood for prolonged periods (up to 12 months or longer).[1]
Mothers with a HIV-1 infection should not breastfeed, because the virus can pass to the baby via the breast milk.[1]
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.[1]
References
1. Full prescribing information [FDA]: Cabenuva (cabotegravir + rilpivirine) [PDF]
ViiV Healthcare, Jan 2021
2. ViiV Healthcare announces FDA approval of Cabenuva (cabotegravir, rilpivirine), the first and only complete long-acting regimen for HIV treatment
ViiV Healthcare, Jan 2021
3. ViiV Healthcare announces first global regulatory approval of Cabenuva; the first complete, long-acting, regimen for the treatment of HIV
ViiV Healthcare, Mar 2020
4. Cabenuva product information sheet
TGA, Feb 2021



Reviews
There are no reviews yet.