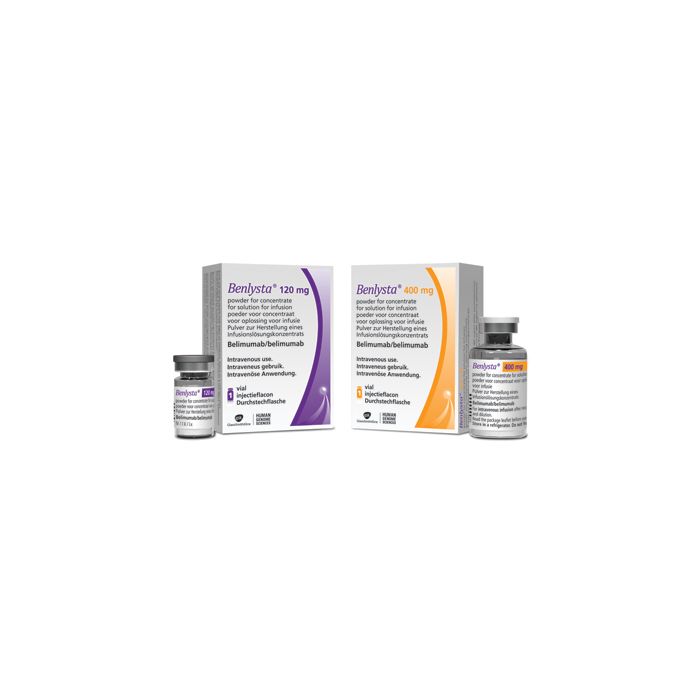
Benlysta (belimumab) for sale – Buy Benlysta (belimumab) Online
What is Benlysta (belimumab) for?
Benlysta (belimumab) is a B-lymphocyte stimulator (BLyS)-specific inhibitor indicated to treat:[1,2]
- Children aged 5 years and older with active, autoantibody-positive, systemic lupus erythematosus (SLE; lupus)
- Adult patients with active lupus nephritis (LN)
It is taken in combination with standard of care therapies and works by suppressing the immune system.[1,2]
It is available as 120 mg in a 5-mL single-use vial and 400 mg in a 20-mL single-use vial for infusion (drip) into a vein. It is also available as a pre-filled pen and pre-filled syringe (200 mg) for injection under the skin.[1,2]
How does Benlysta (belimumab) work?
Approximately 50% of people with the autoimmune disease systemic lupus erythematosus (SLE) will develop lupus nephritis (LN). LN is an inflammation of the kidneys, which causes irreversible kidney damage and significantly increases the risk of kidney failure, cardiac events, and death.[3]
B cells of the immune system play an important role in the inflammation seen in SLE. Normally, B cells make antibodies which help to fight infections. In people with lupus, so-called autoreactive B cells (cells that react against the body) are active longer in the body than normally and produce antibodies that will attack the body’s own cells and organs.[3]
Belimumab works by inhibiting B-lymphocyte stimulator (BLyS), which is a protein that helps B cells live longer. By attaching to the BLyS protein, belimumab makes sure it cannot help B cells, including autoreactive B cells, survive.[1-3]
Where has Benlysta (belimumab) been approved?
Benlysta (belimumab) was approved as add-on therapy by:
- The Food and Drug Administration (FDA), USA:
- On 9 March 2011 as intravenous (IV) formulation for adults with lupus.[4]
- On July 21, 2017 as subcutaneous (SC) formulation for adults with lupus.[5]
- On April 26, 2019 as IV formulation for children aged ≥5 years with lupus.[6]
- On December 17, 2020 as IV and SC formulation for adults with active LN.[7]
- The European Medicines Agency (EMA), Europe:
Benlysta (belimumab) was approved by the FDA under Priority Review for the treatment of lupus. The medicine was granted Breakthrough Therapy Designation and Priority Review for the treatment of adult patients with active LN.[4,7]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Benlysta (belimumab) taken?
There are two ways to take Benlysta (belimumab):[1,2]
- Via subcutaneous self-injection at home for adults with SLE and/or LN:
- A standard dose of 200 mg given once weekly
- Intravenous infusion given by a healthcare provider for adults with SLE and/or LN and for children (aged ≥5 years) with SLE.
- A standard dosage regimen is 10 mg/kg every 2 weeks for the 3 three doses. IV treatment is given over approximately one hour.
- After this, this dose is given every 4 weeks
Benlysta (belimumab) is used in combination with standard of care treatment.[1,2]
Ask your doctor which treatment option is right for you. It is possible for adults to switch from IV treatment to at-home administration.[1,2]
Complete information about Benlysta (belimumab) dosage and administration can be found in the official prescribing information listed in our references section.[1,2]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Benlysta (belimumab)?
Common adverse reactions
The most common side effects ( ≥5% of patients) listed in the prescribing information include:[1,2]
- High temperature or fever (pyrexia)
- Low white blood cell count (can be seen in blood tests)
- Nose, throat or stomach infection
- Pain in hands or feet
- Migraine
- Depression
- Injection site reactions
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1,2]
- Increased risk of infections, including a rare but serious brain infection called progressive multifocal leukoencephalopathy (PML)
Use in a specific population
It is not known whether Benlysta (belimumab) is harmful for a fetus. Women of childbearing potential must use effective contraception during treatment and for at least 4 months after the last treatment. [1,2]
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.[1,2]
References
1. Full prescribing information [FDA]: Benlysta (belimumab) [PDF]
GSK, Mar 2011
2. Full prescribing information [EMA]: Benlysta (belimumab) [PDF]
GlaxoSmithKline (GSK), May 2011
3. Living with Lupus
Benlysta.com, cited Feb 2021
4. FDA approves GSK’s BENLYSTA as the first medicine for adult patients with active lupus nephritis in the US
GSK, Dec 17, 2011
5. GSK receives FDA approval for a new self-injectable formulation of Benlysta (belimumab) for systemic lupus erythematosus
GSK, July 21, 2017
6. GSK receives US approval of Benlysta for intravenous use in children with lupus aged five years and above
GSK, April 26, 2019
7. FDA approves GSK’s BENLYSTA as the first medicine for adult patients with active lupus nephritis in the US
GSK, Dec 17, 2020
8. GlaxoSmithKline and Human Genome Sciences receive European authorisation for Benlysta® (belimumab)
GSK, July 14, 2011
9. GSK receives European marketing authorisation for self-injectable formulation of Benlysta for the treatment of systemic lupus erythematosus
GSK, Nov 13, 2017
10. GSK receives positive CHMP opinion for intravenous Benlysta in children with lupus aged five years and above
GSK, Sept 20, 2019


Reviews
There are no reviews yet.