Emgality (galcanezumab) for sale – Buy Emgality (galcanezumab) Online
What is Emgality (galcanezumab) for?
Emgality (galcanezumab) is a calcitonin-gene related peptide antagonist indicated for:[1]
- The preventive treatment of migraine in adults
- Treating episodic cluster headache in adults
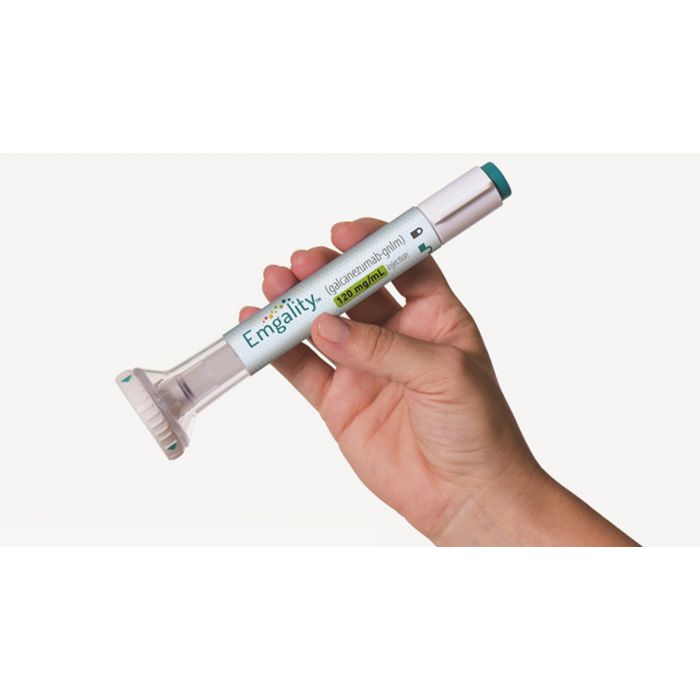
It is available as a single-dose prefilled pen or syringe containing 120 mg/mL of galcanezumab.[1]
How does Emgality (galcanezumab) work?
The active ingredient in Emgality, galcanezumab, is an antibody (a type of protein) that blocks the activity of calcitonin gene-related peptide (CGRP). CGRP is a substance in the brain that is thought to play role in migraine. People with migraine have increased levels of CGRP, which causes inflammation and pain in the nervous systems of people with migraine episodes.[2]
By blocking CGRP binding to the receptor, Emgality might stop the symptoms of migraine.[2]
Where has Emgality (galcanezumab) been approved?
Emgality (galcanezumab) was approved by:
- Food and Drug Administration (FDA), USA:
- European Medical Agency (EMA) on November 14, 2018 to prevent migraine in adults who have migraines at least 4 days a month.[5]
- Therapeutic Goods Administration (TGA), Australia on September 10, 2019 to prevent migraine in adults.[6]
- Health Canada onOctober 2, 2019 to prevent migraine in adults with at least 4 migraine days per month.[7]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Emgality (galcanezumab) taken?
The standard dosage is:[1]
- Starting dose: 240 mg (two injections) once as a loading dose
- Followed by monthly doses of 120 mg
Injections are given under the skin (subcutaneous).[1]
You can self-administer this medicine, after you have received proper training by a healthcare professional.[1]
You can inject in your belly or thighs. Another person can inject in the back of your arm or buttocks.[1]
Leave the pen (with the cap on) at room temperature for 30 minutes before injecting. Do not microwave the pen, run it under hot water, shake it, freeze it, or leave it in direct sunlight.[1]
Complete information about Emgality (galcanezumab) dosage and administration can be found in the official prescribing information listed in our references section.1
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Emgality (galcanezumab)?
Common adverse reactions
The most common side effects (≥2% of patients) listed in the prescribing information include:[1]
- Injection site reactions
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.[1]
References
1. Prescribing Information (FDA): Emgality (galcanezumab-gnlm) [PDF]
Eli Lilly and Company, 2018
2. What is Emgality?
Emgality.com, cited Sep 2021
3. Lilly’s Emgality™ (galcanezumab-gnlm) Receives U.S. FDA Approval for the Preventive Treatment of Migraine in Adults
Lilly press release, Sep 2018
4. FDA approves first treatment for episodic cluster headache that reduces the frequency of attacks
FDA press release, Jun 2019
5. Emgality
EMA, Nov 2018
6. Australian Public Assessment Report Emgality
TGA, Sep 2019
7. EMGALITY™ (galcanezumab) now available in Canada for preventive treatment of migraine
Lilly press release, Oct 2019



Reviews
There are no reviews yet.