Stelara (ustekinumab) for sale – Buy Stelara (ustekinumab) online
What is Stelara (ustekinumab) for?
Stelara (ustekinumab) is an interleukin (IL)-12 and IL-23 antagonist (immunosuppressant) indicated for the treatment of:[1]
- moderate or severe plaque psoriasis in adults and children aged 6 years and older, whose condition has not improved with, or who cannot use, other systemic (whole-body) psoriasis treatments or phototherapy (treatment using ultraviolet light alone or with pills).
- active psoriatic arthritis in adults, with or without another medicine called methotrexate.
- moderately to severely active Crohn’s disease in adults, after other medicines have been tried without success
- moderately to severely active ulcerative colitis in adults, after other medicines have been tried without success
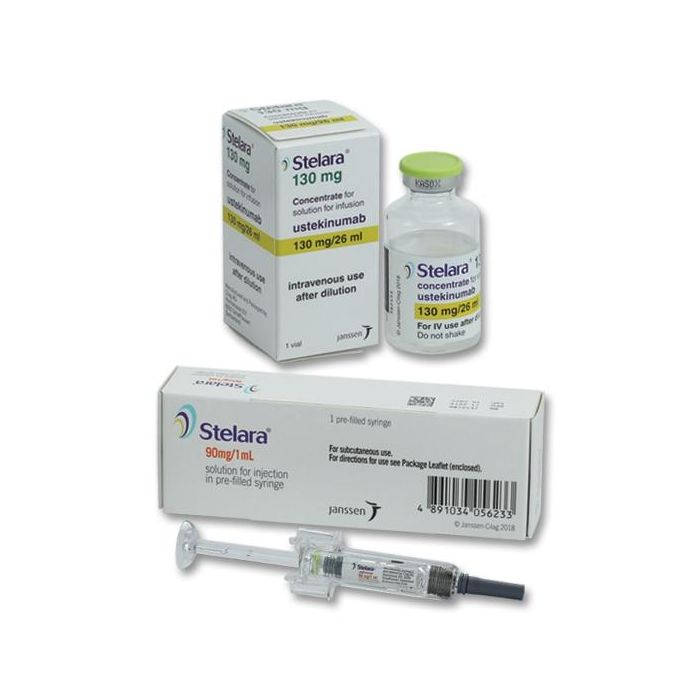
It is available as a single-dose prefilled syringe, containing 45 mg or 90 mg ustekinumab or single-dose vials containing 45 mg of ustekinumab for under the skin injection (subcutaneous use). It is also available in single-dose vial form containing 130 mg ustekinumab for injection into a vein (intravenous use).[1]
How does Stelara (ustekinumab) work?
Ustekinumab is a human monoclonal antibody, a type of protein, that binds to the cytokines interleukin 12 (IL-12) and IL-23. Cytokines play a role in signaling in a cell, especially in inflammation. Dysregulated production of IL-12 and IL-23 were found to be very important in the development of specific diseases, such as psoriasis, psoriatic arthritis, ulcerative colitis and Crohn’s disease.[2]
By binding to IL-12 and IL-23, ustekinumab will block their action, and thereby slow down inflammation.[1,2]
Where has Stelara (ustekinumab) been approved?
Stelara (ustekinumab) was approved by:
- The European Medicines Agency (EMA), Europe:
- On January 15, 2009 for adults with plaque psoriasis.[3]
- On September 23, 2013 for adults active psoriatic arthritis.[4]
- On June 29, 2015, for adults with moderately to severely active Crohn’s disease.[5]
- On November 11, 2016, for adolescents with moderate to severe plaque psoriasis.[6]
- On September 4, 2019, for adults with moderately to severely active ulcerative colitis.[7]
- On January 15, 2020 for pediatric patients with moderate to severe plaque psoriasis.[8]
- The Food and Drug Administration (FDA), USA:
- On September 25, 2009 for adults with plaque psoriasis.[9]
- On September 23, 2013 for adults active psoriatic arthritis.[10]
- On September 26, 2016 for adults with moderately to severely active Crohn’s disease.[11]
- On October 13, 2017 for adolescents with moderate to severe plaque psoriasis.[12]
- On October 21, 2019 for adults with moderately to severely active ulcerative colitis.[13]
- On July 30, 2020 for pediatric patients with moderate to severe plaque psoriasis.[14]
Stelara (ustekinumab) has been granted Orphan Drug designation by the FDA for treatment of type 1 diabetes mellitus patients with residual beta-cell function in November 2010, for treatment of pediatric Crohn’s disease in May 2016, for treatment of pediatric ulcerative colitis in February 2017, and for treatment of pediatric systemic lupus erythematosus in July 2017.[14]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Stelara (ustekinumab) taken?
Plaque Psoriasis
The standard dosage is:[1]
- For patients weighing 100 kg or less: 45 mg given by under the skin (subcutanous) injection initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients weighing more than 100 kg, the recommended dose is 90 mg subcutaneously initially and 4 weeks later, followed by 90 mg every 12 weeks.
Psoriatic Arthritis
The standard dosage is:[1]
- 45 mg given by under the skin (subcutaneous) injection initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients with co-existent moderate-to-severe plaque psoriasis weighing more than 100 kg: 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
Crohn’s Disease
A starting dose is given via intravenous infusion using a body weight-based regimen listed in the prescribing information, followed by a maintenance dosing schedule of a 90 mg subcutaneous maintenance injection every 8 weeks.[1]
Ulcerative Colitis
A single body weight-based dose is given via intravenous infusion, followed by a maintenance dosing schedule of a 90 mg subcutaneous maintenance injection every 8 weeks.[1]
Complete information about Stelara dosage and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Stelara (ustekinumab)?
Common adverse reactions
The most common adverse reactions (≥3% of patients) listed in the prescribing information include:[1]
- Headache
- Nasopharyngitis (inflammation of the nose and throat)
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Increased risk of infections (bacterial, fungal, and viral)
- Decreased activity of your immune system and increased risk for certain types of cancer
Use in a specific population
It is not known whether Stelara (ustekinumab) can cause fetal harm when given to a pregnant woman; it is advised to avoid pregnancies and breastfeeding.[1]
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.[1]
References
1. Full prescribing information [EMA]: Stelara (ustekinumab) [PDF]
Janssen, Jan 2009
2. Targeting IL-23 in psoriasis: current perspectives
Fotiadou C, Psoriasis (Auckl.), Jan 4, 2018
3. Stelara (ustekinumab) product page
EMA, Jan 15, 2009
4. STELARA® Receives European Commission Approval For Treatment Of Active Psoriatic Arthritis
Press release Sept 23, 2013
5. European Commission Approves Stelara® (Ustekinumab) For Treatment Of Adults With Moderately To Severely Active Crohn’s Disease
Press release June 29, 2015
6. STELARA® Receives European Commission Approval For Treatment Of Adolescents With Moderate-To-Severe Psoriasis In Europe
Press release Nov 11, 2016
7. European Commission Approves Expanded Use of Janssen’s STELARA® (ustekinumab) for the Treatment of Moderately to Severely Active Ulcerative Colitis in the European Union
Press release Sept 4, 2019
8. Stelara-H-C-958-II-0073 : EPAR – Assessment Report – Variation [PDF]
EMA, Jan 15, 2020
9. FDA Approves Stelara (ustekinumab) to Treat Psoriasis
Press release Sept 25, 2009
10. STELARA® (Ustekinumab) Receives FDA Approval To Treat Active Psoriatic Arthritis
Janssen press release, Sept 23, 2013
11. FDA Approves Stelara (ustekinumab) for Treatment of Adults with Moderately to Severely Active Crohn’s Disease
Press release Sept 26, 2016
12. Janssen Announces U.S. FDA Approval of STELARA® (Ustekinumab) For The Treatment Of Adolescents With Moderate To Severe Plaque Psoriasis
Janssen press release, Oct 13, 2017
13. Janssen Announces U.S. FDA Approval of STELARA® (ustekinumab) for the Treatment of Adults with Moderately to Severely Active Ulcerative
Jannsen press release, Oct 21, 2019
14. FDA Approves Stelara (ustekinumab) for Treatment of Pediatric Patients with Moderate to Severe Plaque Psoriasis
Press release July 30, 2020





Reviews
There are no reviews yet.