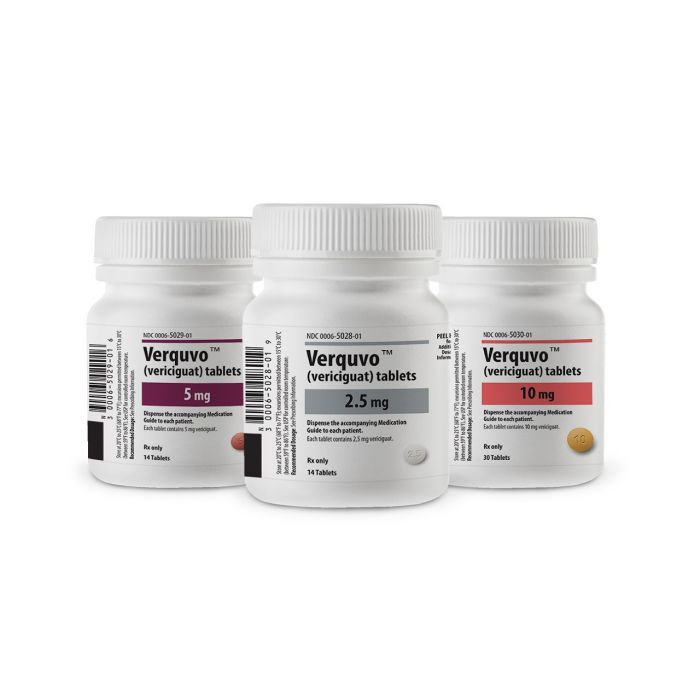
Verquvo (vericiguat) for sale – Buy Verquvo (vericiguat) online
What is Verquvo (vericiguat) for?
Verquvo (vericiguat) is a soluble guanylate cyclase (sGC) stimulator indicated for adults who are having symptoms of their chronic (long-lasting) heart failure, who have had a recent hospitalization or the need to receive intravenous (IV) medicines and have an ejection fraction (amount of blood pumped with each heartbeat) of less than 45%:[1]
- to reduce the risk of dying and
- to reduce the need to be hospitalized
It is available in tablet form, each containing either 2.5 mg, 5 mg or 10 mg of vericiguat.[1]
How does Verquvo (vericiguat) work?
Vericiguat is a stimulator of soluble guanylate cyclase (sGC). SGC is an important enzyme made in the body that causes the blood vessels to become wider (vasodilation). It can bind to nitric oxide (NO), and upon binding, it causes the production of intracellular cyclic guanosine monophosphate (cGMP). cGMP plays a role in the regulation of vascular tone, cardiac contractility, and cardiac remodeling.[2,3]
Heart failure happens when the body’s NO production is too low and the activity of sGC is decreased. As a result the heart is weak and cannot pump enough blood through the body.[2,3]
By stimulating sGC directly, vericiguat lowers the levels of intracellular cGMP, which causes the vessels to relax and to widen.[2,3]
Where has Verquvo (vericiguat) been approved?
Verquvo (vericiguat) was approved to reduce the risk of cardiovascular death and heart failure by:
- The Food and Drug Administration (FDA), USA on January 19, 2021.[3]
- The Pharmaceuticals and Medical Devices Agency (PMDA), Japan on June 23, 2021
- The European Medicines Agency (EMA) on July 21, 2021.[4]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Verquvo (vericiguat) taken?
The standard dosage is:[1]
- 2.5 mg tablets taken orally 1 time each day with food
- Approximately every 2 weeks, the dose of should be doubled to reach the target maintenance dose of 10 mg once daily, to find the best dose for you.
If you miss a dose, take the missed dose as soon as you remember on the same day of the missed dose. Do not take 2 doses of Verquvo (vericiguat) on the same day to make up for a missed dose.[1]
Warning: do not take Verquvo (vericiguat) if you are taking another sGC stimulator. Ask your healthcare provider if you are not sure if you are taking an sGC medicine.[1]
Complete information about Verquvo (vericiguat) dosage (modifications) and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Verquvo (vericiguat)?
Common adverse reactions
The most common side effects ( ≥5% of patients) listed in the prescribing information include:[1]
- Low blood pressure (hypotension)
- Low red blood cells (anemia)
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Severe breathing problems (respiratory failure)
- Internal bleedings (hemorrhage)
- Blood clots (thrombosis)
Use in a specific population
Verquvo (vericiguat) can be fatal for a fetus, it is advised to avoid breastfeeding or pregnancies. Women of reproductive potential should use an effective method of contraception during treatment and for at least 3 weeks after the last dose.1
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.[1]
References
1. Full prescribing information [FDA]: Verquvo (vericiguat) [PDF]
Merck, Jan 2021
2. The efficacy and safety of soluble guanylate cyclase stimulators in patients with heart failure
Zheng X, et al., Medicine (Baltimore), Oct 2018
3. Merck Announces U.S. FDA Approval of VERQUVO® (vericiguat)
Merck, Jan 20, 2021
4. VERQUVO® (vericiguat) Approved in the European Union
Merck, Jul 2021




Reviews
There are no reviews yet.